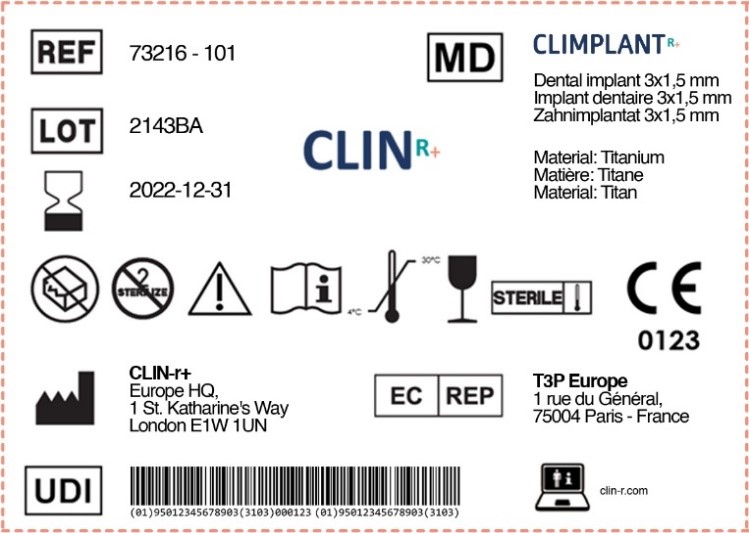
Medical Device Labelling Requirements Canada . There are also several additional requirements.sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in.
from gbu-taganskij.ru
sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in.20 if a medical device consists of or contains software, the software shall be designed to perform as intended by the. There are also several additional requirements.
EU MDR 2017/745 Medical Device Labeling Compliance, 55 OFF
Medical Device Labelling Requirements Canada How to complete the application for a new medical device licence/medical device licence.sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. There are also several additional requirements.20 if a medical device consists of or contains software, the software shall be designed to perform as intended by the.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Labelling Requirements Canada 62.31 (1) the provisions of these regulations — other than this section and sections 44 to 62.2, and 62.32.sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. How to complete the application for a new medical device licence/medical device licence. There are also several additional requirements. Web. Medical Device Labelling Requirements Canada.
From mungfali.com
Medical Device Labeling Symbols Medical Device Labelling Requirements Canada How to complete the application for a new medical device licence/medical device licence. 62.31 (1) the provisions of these regulations — other than this section and sections 44 to 62.2, and 62.32.20 if a medical device consists of or contains software, the software shall be designed to perform as intended by the. There are also several additional requirements.. Medical Device Labelling Requirements Canada.
From www.freseniusmedicalcare.com
Medical device regulation Fresenius Medical Care Medical Device Labelling Requirements Canada 62.31 (1) the provisions of these regulations — other than this section and sections 44 to 62.2, and 62.32. There are also several additional requirements. How to complete the application for a new medical device licence/medical device licence.sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. Web. Medical Device Labelling Requirements Canada.
From www.vrogue.co
Canadian Device Labeling Requirements Ce Mark Package vrogue.co Medical Device Labelling Requirements Canada How to complete the application for a new medical device licence/medical device licence.20 if a medical device consists of or contains software, the software shall be designed to perform as intended by the.sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. There are also several. Medical Device Labelling Requirements Canada.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 55 OFF Medical Device Labelling Requirements Canada 62.31 (1) the provisions of these regulations — other than this section and sections 44 to 62.2, and 62.32. There are also several additional requirements. How to complete the application for a new medical device licence/medical device licence.sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. Web. Medical Device Labelling Requirements Canada.
From flamlabelthema.netlify.app
Medical Device Test Method Validation Template Medical Device Labelling Requirements Canada20 if a medical device consists of or contains software, the software shall be designed to perform as intended by the.sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. How to complete the application for a new medical device licence/medical device licence. 62.31 (1) the provisions. Medical Device Labelling Requirements Canada.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Labelling Requirements Canada How to complete the application for a new medical device licence/medical device licence.sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in.20 if a medical device consists of or contains software, the software shall be designed to perform as intended by the. 62.31 (1) the provisions. Medical Device Labelling Requirements Canada.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Labelling Requirements Canada How to complete the application for a new medical device licence/medical device licence.20 if a medical device consists of or contains software, the software shall be designed to perform as intended by the. There are also several additional requirements.sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical. Medical Device Labelling Requirements Canada.
From mungfali.com
FDA Medical Device Label Symbols Medical Device Labelling Requirements Canada How to complete the application for a new medical device licence/medical device licence. There are also several additional requirements. 62.31 (1) the provisions of these regulations — other than this section and sections 44 to 62.2, and 62.32.20 if a medical device consists of or contains software, the software shall be designed to perform as intended by the.. Medical Device Labelling Requirements Canada.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 55 OFF Medical Device Labelling Requirements Canada There are also several additional requirements. How to complete the application for a new medical device licence/medical device licence.sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in.20 if a medical device consists of or contains software, the software shall be designed to perform as intended. Medical Device Labelling Requirements Canada.
From bchealth.com
Reading and Checking Prescription Medication Labels from Canadian Medical Device Labelling Requirements Canada 62.31 (1) the provisions of these regulations — other than this section and sections 44 to 62.2, and 62.32. How to complete the application for a new medical device licence/medical device licence.20 if a medical device consists of or contains software, the software shall be designed to perform as intended by the. There are also several additional requirements.. Medical Device Labelling Requirements Canada.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Device Labelling Requirements Canada 62.31 (1) the provisions of these regulations — other than this section and sections 44 to 62.2, and 62.32.sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in.20 if a medical device consists of or contains software, the software shall be designed to perform as intended. Medical Device Labelling Requirements Canada.
From www.greenlight.guru
Medical Device Labeling Definition & Requirements Medical Device Labelling Requirements Canada There are also several additional requirements.sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in.20 if a medical device consists of or contains software, the software shall be designed to perform as intended by the. 62.31 (1) the provisions of these regulations — other than this. Medical Device Labelling Requirements Canada.
From www.orielstat.com
Understanding FDA and EU Medical Device Labeling Requirements Oriel Medical Device Labelling Requirements Canada 62.31 (1) the provisions of these regulations — other than this section and sections 44 to 62.2, and 62.32. How to complete the application for a new medical device licence/medical device licence. There are also several additional requirements.sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. Web. Medical Device Labelling Requirements Canada.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Labelling Requirements Canadasections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. 62.31 (1) the provisions of these regulations — other than this section and sections 44 to 62.2, and 62.32.20 if a medical device consists of or contains software, the software shall be designed to perform as intended. Medical Device Labelling Requirements Canada.
From www.tuvsud.com
Infographic The Medical Device Regulation TÜV SÜD Medical Device Labelling Requirements Canada20 if a medical device consists of or contains software, the software shall be designed to perform as intended by the.sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. How to complete the application for a new medical device licence/medical device licence. There are also several. Medical Device Labelling Requirements Canada.
From www.doovi.com
15327_FDA Medical Device Regulations Labeling Require... Doovi Medical Device Labelling Requirements Canada20 if a medical device consists of or contains software, the software shall be designed to perform as intended by the. How to complete the application for a new medical device licence/medical device licence. 62.31 (1) the provisions of these regulations — other than this section and sections 44 to 62.2, and 62.32. There are also several additional requirements.. Medical Device Labelling Requirements Canada.
From paragondsi.com
UDI Unique Device Identification for Single and Multiple Uses Medical Device Labelling Requirements Canadasections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. 62.31 (1) the provisions of these regulations — other than this section and sections 44 to 62.2, and 62.32. How to complete the application for a new medical device licence/medical device licence. There are also several additional requirements. Web. Medical Device Labelling Requirements Canada.